
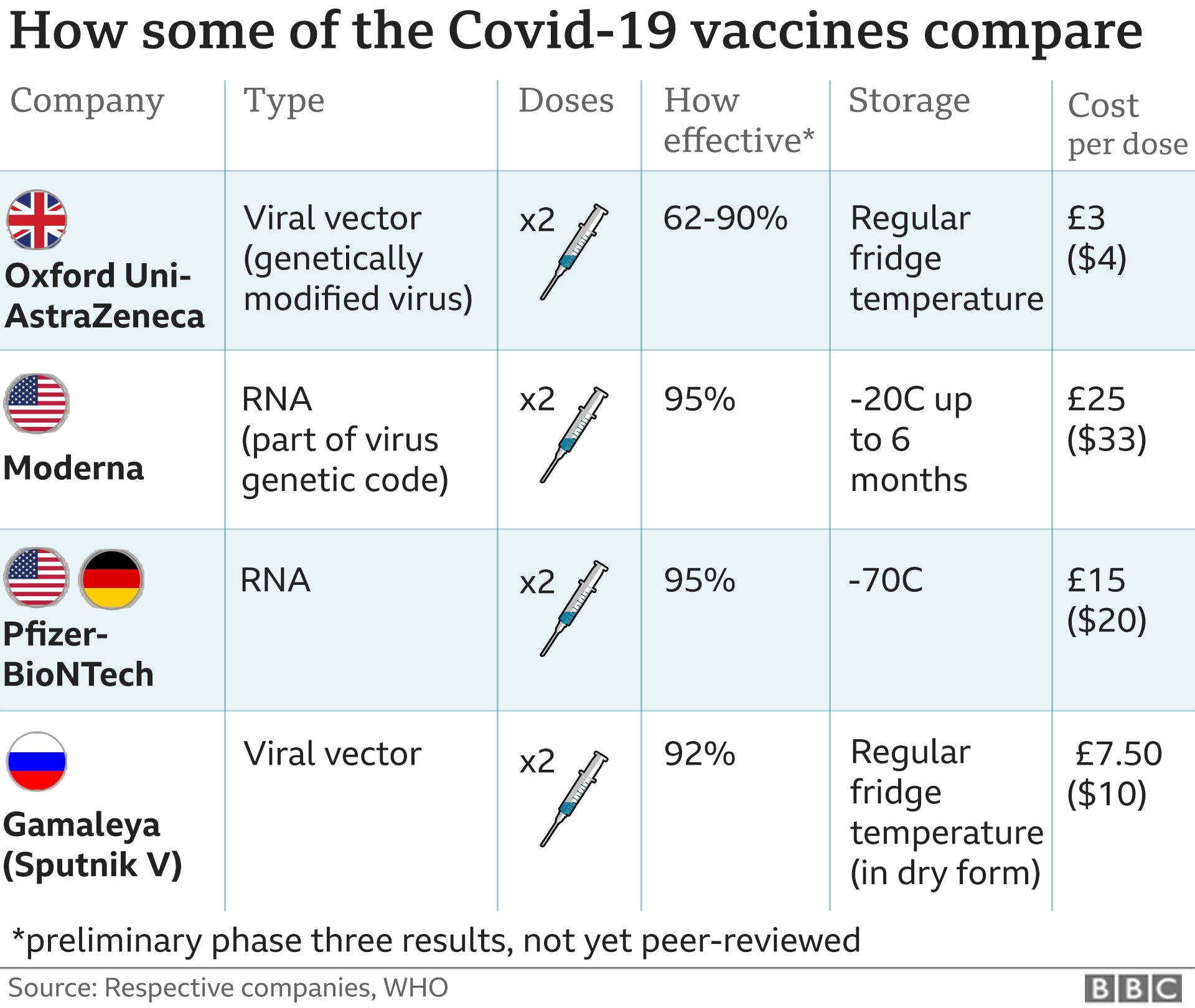
There are two vaccines, made by Moderna and Pfizer, that are both seeking emergency approval in the US. Pfizer's treatment was approved for the British public on Wednesday.
The US Food and Drug Administration (FDA) plans to meet on 10 December to discuss approval for the UK-approved vaccine, which was created through a partnership between Pfizer and BioNTech. They will meet again on 17 December to discuss Moderna's request.
It comes as coronavirus cases continue to balloon across the US, with an average of over 150,000 new cases reported per day. The US has recorded a total of 13.6 million cases and some 270,000 deaths, according to Johns Hopkins University.
Here's what you need to know about when the US will have a vaccine.
What impact does UK approval have on US?
On Wednesday, the UK became the first country to approve the Pfizer vaccine, meaning that mass injections could start next week.
The elderly, as well as medical workers, will be given top priority, following by those with medical issues, then by age.
The UK's approval is expected to place extra pressure on FDA regulators to swiftly approve the vaccine - the CNN's chief medical correspondent, Sanjay Gupta, said it was encouraging that there were no red flags in the UK over the vaccine's safety. American regulators will examine the same data.
The CDC's Covid-incident manager, Dr Henry Walke, said on Wednesday that they had seen the news, and "we are still evaluating that approval in the UK".
The British experience in distribution can also help guide US authorities.
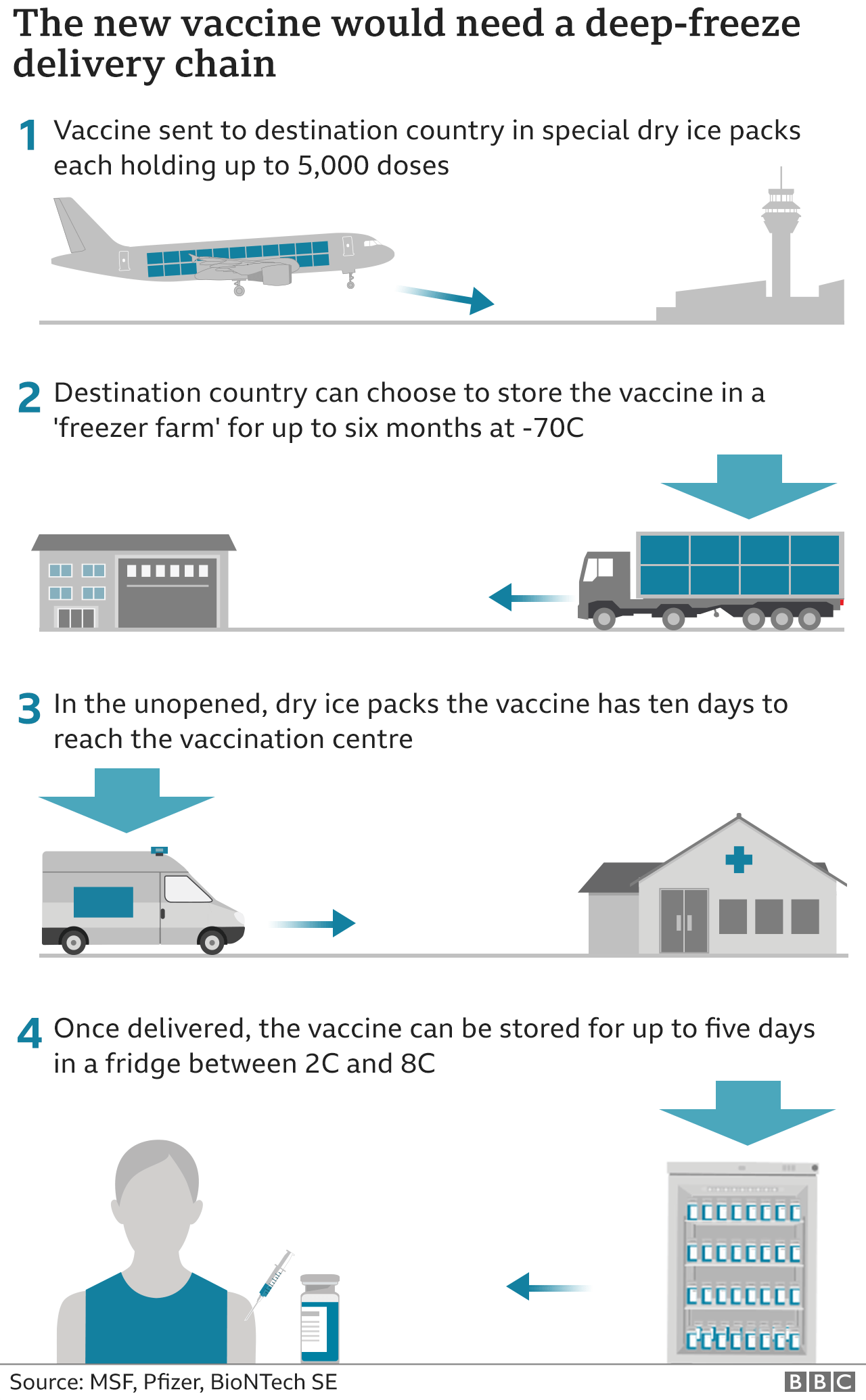
This vaccine must be stored at around -70C (-94 F) and will be transported in special boxes of up to 5,000 doses, packed in dry ice.
Once delivered, it can be kept for up to five days in a fridge. And once out of the fridge it needs to be used within six hours.
Who will get the US vaccine first?
Federal officials at the Centers for Disease Control and Prevention (CDC) agree that the nation's 21 million healthcare workers should be prioritised first, as well the three million elderly Americans living in long-term care homes.
But there is less consensus on how states should distribute it to other groups.
The nation's approximately 87 million essential workers are expected to be next in line for the jab, but it will be up to states to decide which industries to prioritise. Will postal workers and meat-processing factory workers be included, for example?
Moncef Slaoui, who leads the federal government's Operation Warp Speed vaccine distribution programme, said he does not "expect the states to make uniform decisions".
"Some may prefer long-term care facilities or the elderly, while others may prioritise their health care workers. It would be wrong to immunise 18-year-olds first. I hope no one does that. But otherwise it's shades of grey."
Officials say vaccinations for groups that are not at a high risk are expected to take place in the spring of 2021.
There are also ongoing concerns regarding how many Americans are willing to get vaccinated. A recent Gallup poll found that 58% of Americans say they would be willing to get the jab, up from a low of 50% in September.
It also remains to be seen how racial minorities - who are at a higher risk of catching the virus and dying from it - will be prioritised.
Last month, New York's governor decried the federal government's immunisation plan as "discriminatory," saying that distribution infrastructure such as pharmacies and hospitals are less prevalent in black and Latino communities.
None of the vaccines currently under review have been tested on pregnant or nursing women, meaning they will be unlikely to be advised by the government to get vaccinated.
What happens next?
There a re a few key dates coming up.
- 8 Dec - White House summit with vaccine firms
- 10 Dec - FDA could approve Pfizer
- 14 Dec - rollout of Pfizer vaccine could begin this week
- 17 Dec - FDA could approve Moderna
President Trump will host the "Covid-19 Vaccine Summit" at the White House on 8 December.
The event is expected to bring together vaccine manufacturers, drug distributors and government officials and is being seen by experts as an opportunity for the White House to apply pressure on all involved to swiftly make vaccines available in the US.
On Monday, Vice-President Mike Pence told US governors in a conference call that rollout of the vaccine could begin "as soon as the week of December 14".
Pfizer plans to have 6.4 million doses ready for delivery by 19 December. Because two shots are required per person, that is enough for three million people, out of a total US population of 330 million.
By the following week, both Pfizer and Moderna are expected to have produced enough vaccines for another 10 million people. By the end of the month, both companies are anticipated to have produced enough vaccine for 30 million people.
What will the vaccines cost?
In July, the US Department of Health and Human Services announced a $1.95bn (£1.45bn) deal to secure 100 million vaccine doses from Pfizer. The agreement also allows the US government to purchase an additional 500 million doses.
Moderna received nearly $1bn from the US government for coronavirus research. They are set to receive an additional $1.5bn for 100 million doses, according to a deal signed in August.
Billions of dollars have also been promised to other drug companies in the event that they are able to bring additional vaccines to market.
The CDC says that vaccines purchased with taxpayer money will free, but providers may still charge for administering the jab. That fee may be reimbursed by health insurance companies or the Medicaid and Medicare programmes - social safety nets for low income and elderly Americans.
States are also racing to acquire ultra-cold refrigerators that are capable of storing the Pfizer vaccine, which much be kept at extremely low temperatures.
Each refrigerator costs tens of thousands of dollars, and have been harder to find in recent days as hospitals race to purchase them.
"What" - Google News
December 03, 2020 at 12:24AM
https://ift.tt/3lsRk2P
Covid vaccine: What does UK vaccine approval mean for US? - BBC News
"What" - Google News
https://ift.tt/3aVokM1
https://ift.tt/2Wij67R
Bagikan Berita Ini















0 Response to "Covid vaccine: What does UK vaccine approval mean for US? - BBC News"
Post a Comment